Background
The development of therapy-related multiple myeloma (t-MM) is a poorly described entity. Multiple myeloma (MM) is the second most common hematologic malignancy. Despite significant strides in MM treatment over recent decades, identifying modifiable risk factors, optimizing screening methodologies, and identifying potential treatment strategies or targets to address t-MM remains of utmost importance, as this disease continues to pose a significant therapeutic challenge and remains incurable. This retrospective review seeks to explore the incidence and characteristics of t-MM at a medium-sized, academic center.
Methods
We performed a single institution retrospective chart review of patients diagnosed with multiple myeloma between 2008-2023 and evaluated for prior diagnosis of solid malignancy, excluding all skin cancers, lymphomas or myeloproliferative neoplasm (MPN). We collected data for the following: prior treatment for solid malignancy, lymphoma or MPN, lapsed time between prior therapy and development of MM, cytogenetics, prominent subtype present, renal failure and hypercalcemia if present at time of diagnosis, management of t-MM including transplant data, disease progression including relapse status, and survival data.
Results
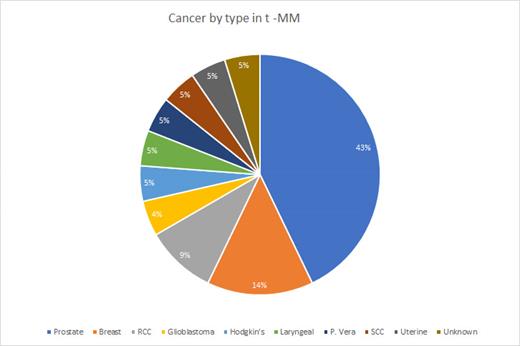
In our study, we identified a subgroup of 17 individuals who had a previous history of solid malignancy, 1 patient with unknown prior solid tumor, 1 patient with history of Hodgkin's lymphoma, and 1 additional patient with history of MPN who received chemotherapy and/or radiation therapy, with 3 patients having MGUS prior to development of MM. Notably, among this population, 70% were male. All patients developed MM following the treatment for a solid malignancy, lymphoma or MPN. The average amount of time from diagnosis of solid tumor, lymphoma or MPN to development of MM was 9.53 years (1-25 years). 50% of the individuals were White Americans, while the remaining 50% were African Americans. A significant majority (85%) of the population had a single primary solid tumor. Among the primary solid tumors, 45% were prostate cancer, 15% were breast cancer, and 10% were renal cell carcinoma (Figure 1). 5 patients received chemotherapy alone without documented prior radiation exposure while 13 had prior radiation exposure for prior solid malignancy. At time of diagnosis, 45% of patients had renal failure with 10% of patients having both renal failure and hypercalcemia. Within the t-MM subtypes, we discovered that 50% had IgA type, with 70% of these individuals exhibiting the IgA Kappa subtype. 45% of the individuals who developed t-MM had the IgG type, with 78% of them with IgG Kappa subtype. 15 (75%) of the patients received transplant and only 2 patients had confirmed relapse with 2 patients unknown and 2 additional patients who passed away prior to starting maintenance therapy following transplant. 4 patients are very early in their disease process and as of yet have not undergo transplant. 55% of our patients are presumed living. The OS for patients with t-MM was 1190 days compared to the average OS of 2326 days in our institutional MM database without t-MM.
Conclusion
Our analysis focused on patients diagnosed with MM who had a prior history of solid primary tumors, lymphoma or MPN treated with chemotherapy and/or radiotherapy in hopes of providing valuable insights into the characteristics and outcomes of individuals who developed MM following treatment for solid tumors, lymphoma or MPN. Our findings highlighted specific risk factors associated with t-MM in this population. Being male and having a history of prostate cancer was associated with higher incidence of development of t-MM. This is likely related to the radiation therapy associated with prostate cancer, as radiation exposure has been a known risk factor for the development of MM. We observed that the predominant subtype of MM among these individuals was IgA kappa, which diverges from the prevalent subtype, IgG kappa, observed in the general population. IgA is known to typically have more cytogenetic abnormalities and overall poorer prognosis, likely contributing to the difference in OS compared to patients with t-MM and MM not associated with prior malignancy. These insights shed light on the unique characteristics and potential influences of treatment-related MM in patients with prior malignancies or MPN.
Disclosures
Kota:Novartis: Honoraria; Kite: Honoraria; Pfizer: Honoraria; Incyte: Research Funding. Cortes:Takeda: Consultancy, Honoraria; Abbvie: Consultancy, Research Funding; Gilead: Consultancy; Pfizer: Consultancy, Research Funding; Biopath Holdings: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Forma Therapuetic: Consultancy; Novartis: Consultancy, Research Funding. Keruakous:BMS: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal